Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 catalyst
catalystHakoda, Teruyuki; Chowdhury, M. A. Z.*; Shimada, Akihiko; Hirota, Koichi
Plasma Chemistry and Plasma Processing, 29(6), p.549 - 557, 2009/12
Times Cited Count:5 Percentile:23.93(Engineering, Chemical)The decomposition of dimethyl sulfide (DMS) at initial concentrations of 4.5-18.0 ppmv in air was studied under electron-beam (EB) irradiation. Doses to decompose 90% of input DMS were 2.5 kGy for 4.5 ppmv, 3.4 kGy for 10.6 ppmv, and 3.9 kGy for 18.0 ppmv. HCOOH, (CH )
) SO, and trace CH
SO, and trace CH OH and (CH
OH and (CH )
) SO
SO were produced as irradiation products in addition to CO
were produced as irradiation products in addition to CO and CO. Application of an O
and CO. Application of an O decomposition catalyst to an irradiated sample gas led to an enhancement in the oxidation of DMS and its products into CO
decomposition catalyst to an irradiated sample gas led to an enhancement in the oxidation of DMS and its products into CO and the decomposition of O
and the decomposition of O . For 10.6 ppmv DMS/air, the mineralization ratio increased from 41% via only EB irradiation to 100% via the combination treatment at 6.3 kGy. The yield of CO
. For 10.6 ppmv DMS/air, the mineralization ratio increased from 41% via only EB irradiation to 100% via the combination treatment at 6.3 kGy. The yield of CO to CO
to CO increased from 5.3% to 87.6% by combination with catalytic oxidation. This combination treatment enables the irradiation energy used to deodorize gas streams containing DMS to be reduced.
increased from 5.3% to 87.6% by combination with catalytic oxidation. This combination treatment enables the irradiation energy used to deodorize gas streams containing DMS to be reduced.
Hakoda, Teruyuki; Shimada, Akihiko; Matsumoto, Kanae*; Hirota, Koichi
Plasma Chemistry and Plasma Processing, 29(1), p.69 - 78, 2009/02
Times Cited Count:6 Percentile:26.91(Engineering, Chemical)Electron beam (EB) technology has an advantage for treating dilute environmental pollutants in gases due to high-density population of active species such as radicals and atoms. In general, OH radicals play an important role of initiating the decomposition and removal of such pollutants. It is quite important to understand the behavior of OH radical production for the development of efficient decomposition/removal processes and the comparison with other purification methods. The number of OH radicals produced in humid N at doses of 2.0-10.0 kGy with dose rates of 0.17-2.55 kGy/s under 1-MeV EB irradiation was indirectly determined using an index of oxidation of CO to CO
at doses of 2.0-10.0 kGy with dose rates of 0.17-2.55 kGy/s under 1-MeV EB irradiation was indirectly determined using an index of oxidation of CO to CO , which has been used in atmospheric chemistry. An experiment under conditions where all OH radicals produced react with CO demonstrated that the concentration of CO
, which has been used in atmospheric chemistry. An experiment under conditions where all OH radicals produced react with CO demonstrated that the concentration of CO increased linearly with doses of 0-10 kGy, and the
increased linearly with doses of 0-10 kGy, and the 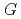 (OH) was estimated as 4.90.
(OH) was estimated as 4.90.
Ostapczuk, A.*; Hakoda, Teruyuki; Shimada, Akihiko; Kojima, Takuji
Plasma Chemistry and Plasma Processing, 28(4), p.483 - 494, 2008/08
Times Cited Count:16 Percentile:53.62(Engineering, Chemical)The application of non-thermal plasma generated by electron beam (EB) was investigated in laboratory scale to study decomposition of polycyclic aromatic hydrocarbons like naphthalene and acenaphthene in flue gas. PAH compounds were treated by EB with the dose up to 8 kGy in dry and humid base gas mixtures. Experimentally established G-values gained 1.66 and 3.72 mol/100 eV for NL and AC at the dose of 1 kGy. NL and AC removal was observed in dry base gas mixtures showing that the reaction with OH radical is not exclusive pathway to initialize PAH decomposition, however in the presence of water remarkably higher decomposition efficiency was observed. As by-products of NL decomposition were identified compounds containing one aromatic ring and oxygen atoms besides CO and CO . It led to the conclusion that PAH decomposition process in humid flue gas can be regarded as multi-step oxidative de-aromatization analogical to its atmospheric chemistry.
. It led to the conclusion that PAH decomposition process in humid flue gas can be regarded as multi-step oxidative de-aromatization analogical to its atmospheric chemistry.
Maehara, Tsunehiro*; Miyamoto, Ippei*; Kurokawa, Kenya*; Hashimoto, Yukio*; Iwamae, Atsushi; Kuramoto, Makoto*; Yamashita, Hiroshi*; Mukasa, Shinobu*; Toyota, Hiromichi*; Nomura, Shinfuku*; et al.
Plasma Chemistry and Plasma Processing, 28(4), p.467 - 482, 2008/08
Times Cited Count:53 Percentile:87.71(Engineering, Chemical) under electron beam irradiation
under electron beam irradiationHakoda, Teruyuki; Matsumoto, Kanae; Mizuno, Akira*; Kojima, Takuji; Hirota, Koichi
Plasma Chemistry and Plasma Processing, 28(1), p.25 - 37, 2008/02
Times Cited Count:22 Percentile:63.83(Engineering, Chemical)The oxidation of xylene and its irradiation byproducts in air using TiO was studied under electron beam (EB) irradiation for the purification of ventilation gases emitted from paint factories. EB irradiation experiments were performed mainly under two different conditions: a TiO
was studied under electron beam (EB) irradiation for the purification of ventilation gases emitted from paint factories. EB irradiation experiments were performed mainly under two different conditions: a TiO pellet layer was placed in an irradiation or non-irradiation space. The results revealed that xylene was decomposed and CO was formed in the gas phase of the irradiation space irrespective of the presence of the TiO
pellet layer was placed in an irradiation or non-irradiation space. The results revealed that xylene was decomposed and CO was formed in the gas phase of the irradiation space irrespective of the presence of the TiO pellets, while CO
pellets, while CO was produced in the gas phase of the irradiation space and on the surface of the TiO
was produced in the gas phase of the irradiation space and on the surface of the TiO pellets. The total CO
pellets. The total CO concentration increased when the pellet layer was in the non-irradiation space. On the other hand, the concentration of CO
concentration increased when the pellet layer was in the non-irradiation space. On the other hand, the concentration of CO produced on the surface of the TiO
produced on the surface of the TiO pellets in the irradiation space was higher than that in a non-irradiation space.
pellets in the irradiation space was higher than that in a non-irradiation space.